Researchers at Oregon Health and Science University (OHSU) used 3D printed miniature Lego-style “bone bricks” that have the potential to heal fractured bone tissue. The researchers’ tiny hollow block is only the size of a small flea, and can be used as a scaffold, allowing both hard and soft tissues to grow on it. In addition, the stackable nature of the modules allows them to be interlocked like building blocks, providing scalability and thousands of potential geometric configurations. Ultimately, the Oregon team aims to expand the scale of the technology and use organs made in the microcage production laboratory for human transplantation.
“The scaffold we are applying for a patent is easy to use; Dr. Luiz Bertassoni, associate professor of biomedical engineering at OHSU School of Medicine, said: “It can be stacked like Lego and placed in thousands of different configurations to suit almost any situation. Complexity and size.
3D printed biomaterial scaffold
In recent years, the biological structure of printed scaffolds has become a research hotspot, especially for applications in tissue engineering or regenerative medicine. In addition, advances in 3D printing technology have enabled patient-specific implantable structures to be designed in a more extensible manner. In some cases, they can even be produced on-site in hospitals. As a result, specialized equipment is no longer required to assemble these complex tissues, thereby reducing the lead time associated with implant production.
Nevertheless, the development of an ideal scaffolding system has proven to be elusive, which is still one of the reasons why the technology has not been more widely adopted in the hospital environment. The ideal tissue support must not only be compatible with the defect-specific architecture, but also allow for the controlled loading of cells, growth factors, and hydrogels. In addition, according to the Colorado team, temporary control of the tissue is essential for the ingrowth of the tissue within the graft material.
3D printed bone bricks by the Oregon team
Conventionally, plastic surgeons repair complex fractures by implanting metal rods or metal plates into the patient’s body to stabilize the bones. Only in the later stages of the process, biocompatible scaffold materials containing powders or pastes are used to promote healing. On the other hand, the Oregon team has developed a novel scaffolding system that can accurately place hollow blocks containing a small amount of growth factor gel in the most needed position.
The co-author of the study, Dr. Ramesh Subbiah, a postdoctoral fellow in the Bertassoni OHSU laboratory, explained: “The 3D printed microcage technology improves healing by stimulating the growth of the right cell types at the right place and at the right time. Different growth factors can be placed in Within each block, we can repair tissues more accurately and faster.”
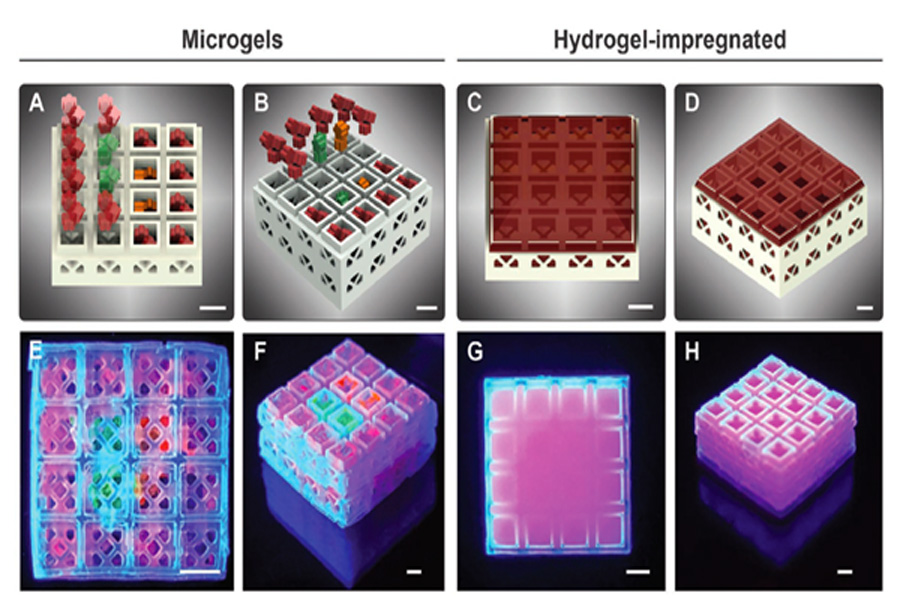
The team’s miniature cages are hollow inside, allowing them to load cargoes of different biogel compositions in a controlled manner and create scaffolds with spatially deterministic indicators. As a proof of concept, the 3D team printed many blocks containing micro-particle hydrogels containing various growth factors. The results showed that the cells entered the scaffold in a fast and controlled manner, thereby accelerating the formation and healing process of new tissue.
3D printing modular design for testing researchers
The team used β-tricalcium phosphate ceramics and a photolithography-based ceramic manufacturing (LCM) 3D printing technology to create many modular miniature cage systems. The block size produced by this process is 3.375 mm3, the hollow size is 1.5×1.5×1.5 mm, and the wall thickness is 230–560 µm. In short, using sample tiles, researchers can easily create various shapes while maintaining a consistent outline around them. The Oregon team studied four-layer 4×4 blocks, and a total of 29,413 configurations can be configured, thus highlighting the potential of this technology in patient-customized bone stents. To illustrate the suitability of its method for use with other rigid polymer materials, many other bricks were created using methacrylate-based resins, which are often used in similar regeneration procedures.
In order to demonstrate the potential of scaffolding in regenerative applications, the team used digital light processing (DLP) 3D printing technology to create a series of special-shaped products with five-point flower-like geometric shapes. Then different combinations of human recombinant growth factors were manually loaded into modules of various shapes. After stacking, the strength of the two-layer blocks was significantly reduced to 13.8 MPa, but this was still much higher than the reported average jawbone strength of 3.9 MPa.
In addition, further experiments found that the growth factor-filled blocks placed near the repaired rat bones caused blood vessel growth three times that of traditional scaffolding materials. As a result, the researchers concluded that although their method has been optimized for hard tissue repair, the concept may be applicable to other tissue regeneration applications. Through extensive research, the Oregon team believes that the modular approach can be used to repair more complex fractures in larger animals, and even for human transplants.
Additive manufacturing and bone repair
Many researchers from academic institutions around the world are already exploring the concept of 3D bioprinted bone implants. For example, researchers at the University of Manchester have developed bone bricks similar to the Oregon team. The device was created to meet emergency medical needs in Syrian refugee camps.
At the same time, researchers at Delft University of Technology have designed and printed porous titanium bone implants with antibacterial properties. The synergistic antibacterial behavior of the graft may result in a new type of implant that allows the patient to maintain a minimum of life.
On the other hand, scientists at Texas A&M University combined 3D printing, biomaterial engineering and stem cell biology to create a new and more efficient facial bone graft. The highly osteogenic scaffold not only promotes the growth of bone cells, but also serves as a solid platform for bone regeneration.





